All medicines may cause side effects
The first thing to remember is that no medicine is completely free from side effects. Patient information leaflets always list those that were observed during research studies and after the medicine became available on the market. However, this does not mean that every person who takes the medicine will experience them.
It is doctor’s role is to weigh the potential risks and benefits for each individual. That is why treatment for obesity should always be prescribed by qualified healthcare professionals after a thorough assessment of the patient.
Are they really safe?
Overall, these medicines have a safety profile considered to be acceptable, which helps explain their current popularity. Nevertheless, their safety must always be evaluated on an individual basis.
They work by mimicking hormones produced in the gut (GLP-1 or GIP) that are released after meals. Because of this mechanism, they generally do not cause complex drug interactions.
The most common side effects
The side effects most often reported are related to digestion:
-
Nausea (vomiting is rare).
-
Acid reflux or heartburn.
-
Constipation, or in some people, diarrhoea.
The good news is that these effects are usually dose-related and tend to improve over time.
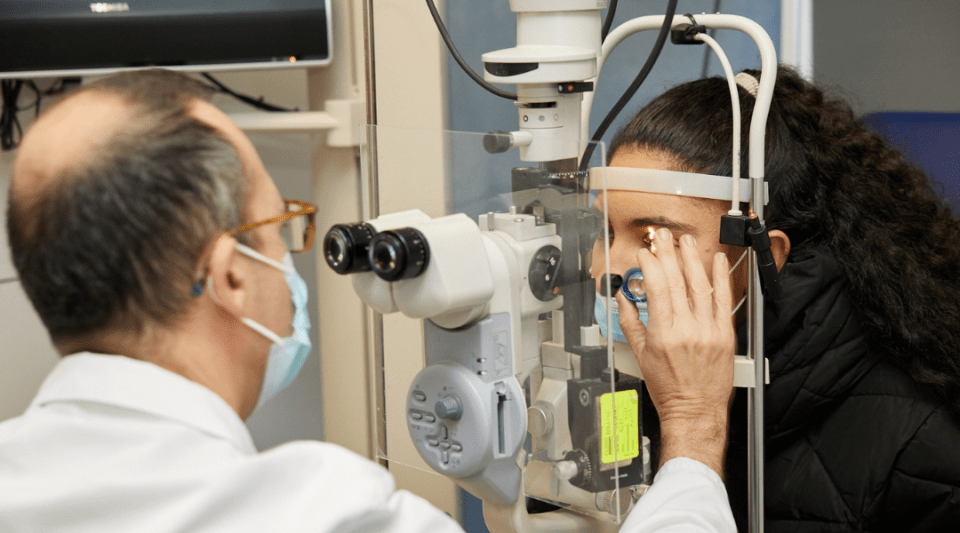
What about vision?
There are two important situations to consider:
-
In people with poorly controlled diabetes and existing retinopathy, a sudden drop in blood sugar levels can temporarily worsen vision. This can happen with semaglutide, but also with other treatments such as insulin.
-
More recently, the European Medicines Agency (EMA) has warned of a very rare risk of optic neuritis in patients treated with semaglutide: around 1 case per 1,000 people over 3 years.
The courts are also examining this matter
It is worth noting that in the United States, legal proceedings have been launched concerning these medicines. More than 1,200 collective lawsuits have been filed against GLP-1 medicines, including Ozempic®. Although health authorities continue to consider these serious side effects as rare, the treatments’ growing popularity and widespread use have brought increased legal and media scrutiny. The pharmaceutical company has argued that many of the complaints relate to off-label use, meaning use beyond the indications approved by regulatory bodies.
In summary, in such a large population of patients being treated, even rare side effects can occasionally occur. These medicines have become an important tool in improving health for many people, but their use must always be supervised by healthcare professionals. The decision to start or continue treatment should always be made jointly by the patient and their doctor.
INFORMATION DOCUMENTED BY:
Antonio Jesús Blanco and Ana de Hollanda, endocrinologists at the Endocrinology and Nutrition Department of the Hospital Clínic Barcelona and members of the Diabetes Unit.