19 February 2026
- What is cancer immunotherapy?
- Immunotherapy types
-
CAR-T Therapy
-
TIL Therapy
-
The procedure at the Clínic
-
Team and structure
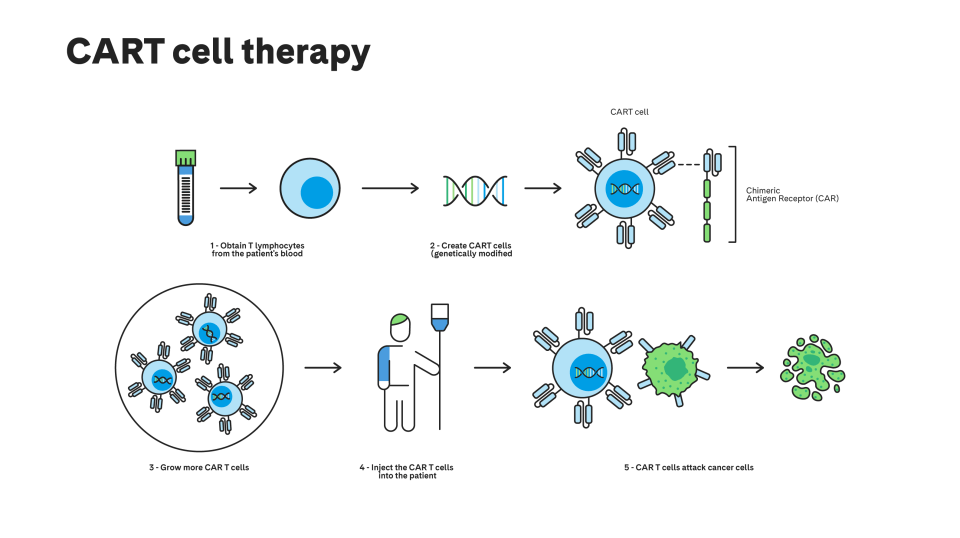
What is CAR-T therapy?
CAR-T (Chimeric Antigen Receptor T-Cell) therapy consists of the use of the patient's own T lymphocytes. A species similar to an antibody is added to these cells via genetic modification that recognises the antigen tumour (a substance called a scFv fragment) and one of the membrane receptors of the T lymphocytes. This is why it is called a "chimeric" receptor.
How is it performed?
The first step in CAR-T therapy is apheresis on the patient. The blood sample collected is inserted into a device called a cellular bioreactor, which separates the T-cells from the rest of the blood cells. After separation, these cells are genetically modified to give them their anti-tumour capacity.
Once modified, the CAR T-cells are reproduced within the bioreactor until the number of cells necessary for the desired effectiveness is obtained. These CAR T-cells are then infused back into the patient to attack the malignant cells.
All patients treated with CAR-T therapy are followed up and monitored. A laboratory analysis is performed to determine parameters such as the CAR T-cell population in the blood; the presence of different antibodies; and levels of some types of immune cells.
This patient follow-up allows specialists to assess the effectiveness of the treatment and make the most appropriate decisions in each situation.
The CAR-T therapy production process must be according to specific “Good Manufacturing Practice” (GMP) standards.
GMP comprises a set of measures to ensure the quality of medicines and protect the patient and manufacturer. For example, there are a set of specifications to ensure the treatment is of a proper composition; its production is homogeneous; its format and dosage are appropriate; and there is no contamination.
Substantiated information by:


Published: 21 September 2023
Updated: 21 September 2023
Subscribe
Receive the latest updates related to this content.
(*) Mandatory fields
Thank you for subscribing!
If this is the first time you subscribe you will receive a confirmation email, check your inbox